Spatiotemporal regulation of mitochondrial bioenergetics and signaling in metastatic cancer
Despite significant progress in determining the molecular and biochemical determinants of the metastatic cascade, we still lack actionable targets for metastatic disease. Filling this gap, our lab focuses on understanding the importance of mitochondria biology in metastatic breast and prostate cancer.
Mitochondria are bioenergetic, biosynthetic, and signaling organelles that have been implicated in various aspects of tumorigenesis and therapy resistance. Indeed, mitochondrial respiration has been linked to oncogene-dependent transformation, metabolic reprogramming, protein translation, tumor repopulation, cell motility and metastasis. In this context, mitochondrial quality control mechanisms are emerging as drivers of metastasis and attractive therapeutic targets.
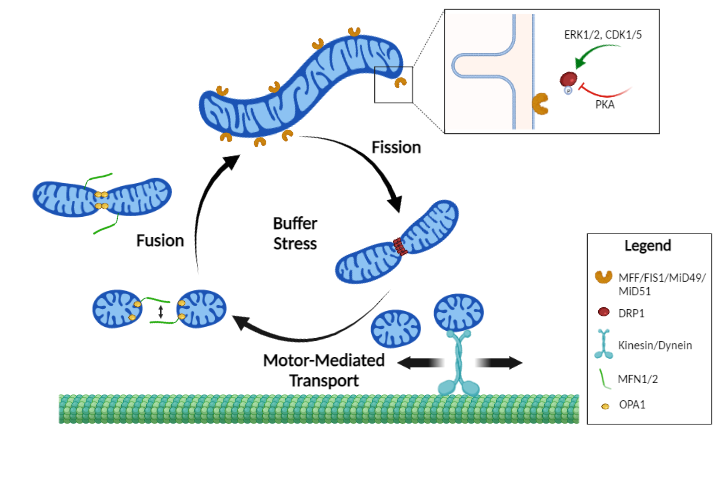
Mitochondrial dynamics in cancer.
A key mechanism by which tumors regulate mitochondrial functions are the collective processes of mitochondrial dynamics. Mitochondrial dynamics refers to spatiotemporal regulation of gross morphology, transport of organelles, inner membrane and cristae morphology, communication between adjacent mitochondria and contact with other organelles such as the endoplasmic reticulum. Current projects look into the functional consequences of mitochondrial shape changes in the metastatic potential of cancer cells.
Deregulated mitochondrial trafficking fuels metastasis.
Syntaphilin (SNPH), the molecular brake anchoring mitochondria to microtubules, is broadly expressed in normal tissues and lost in most cancer types, including breast cancer. Higher levels of SNPH were associated with removal of mitochondria from the cortical cytoskeleton, impaired focal adhesion dynamics and inhibition of tumor cell invasion and metastasis. In patients, SNPH loss in tumors correlated with shortened patient survival. Current projects examine how SNPH is regulated in metastatic cancer, with the long term goal of exploiting this knowledge for therapeutic gain.
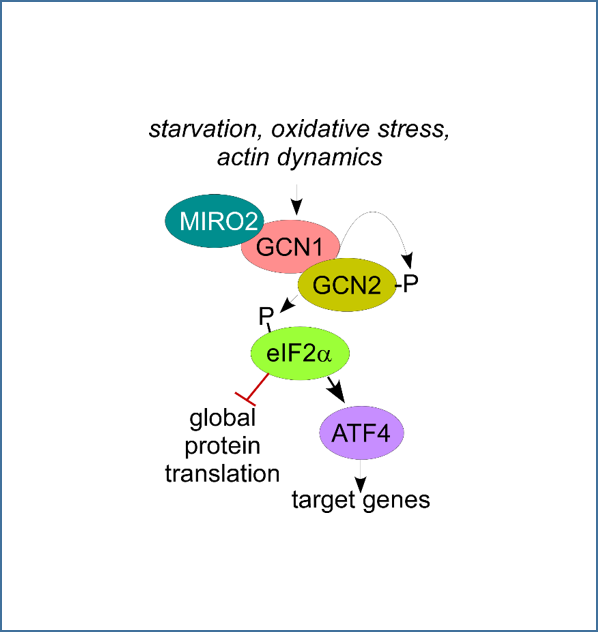
Mitochondria engage the integrated stress response to stimulate metastatic cancer growth.
Our work identified a novel signaling pathway that drives the growth of prostate cancer. This pathway is centered in Mitochondrial Rho GTPase 2 (MIRO2), a small GTPase of the Ras superfamily. MIRO2 binding to GCN1 led to activation of the integrated stress response kinase GCN2, facilitating translation of ATF4, both in vitro and in vivo. MIRO2 is overexpressed in metastatic cancers and higher levels of MIRO2 correlate with poor patient survival. Current projects aim to test the value of MIRO2/GCN1/GCN2 as therapeutic targets in metastatic cancer.
Our lab is supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under award no. W81XWH-21-1-0408 (to M.C. Caino); NIH R35 GM142774 (to M.C. Caino); Cancer League of Colorado AWD-25B6265 (to M.C. Caino), T32 GM007635 (R. Farahani). Previous funding for these projects include ACS IRG-16-184-56 (M.C. Caino), Boettcher Foundation AWD-193249 (M.C. Caino), NCI F31 CA271652 (to D.P. Boulton), T32 GM007635 (M. Furnish, D.P. Boulton), and T32 GM136444 (to R. Lerma). Use of Core Facilities is subsidized by the Cancer Center Support Grant (CSSG) P30 CA046934 to the University of Colorado Cancer Center. CREU summer fellows in our lab were funded by The Cancer League of Colorado; and NCI grant 1R25CA240122.